Introduction
In chemical kinetics, reaction rate constant , also known as rate constant k Or λ is a quantization representation of the chemical reaction rate.
For the Reactant A and Reactant B to be used as a chemical reaction of produce C , the reaction rate can be expressed This form:
k (t) is the reaction rate constant, which will change the temperature change
[x] is assumed The volume of the substance X is in the case where the substance X is occurred in a fixed volume. (When the reaction occurs in a certain range, it can be expressed in the unit area of X.)
index m and n are referred to as the number of reaction stages, depending on the reaction mechanism. It can be determined by experiments.
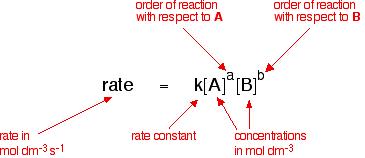
If a reaction can be written, it can also be written as:
Arnicnis equation provides a quantitative basis for reacting the relationship between reaction rates and activation energy.
Chemical reaction rate
The reaction rate is , the chemical reaction is slow, the unit is mol / (L · S) or MOL / (L · min) . The reduction in the concentration of the reactants in units of time or the amount of product concentration is increased. The concentration unit generally uses molar · sup> -1 , time units for seconds, minutes or hours. The chemical reaction is not uniformity: the reaction rate is divided into an average rate (average reaction rate at a time interval) and the instantaneous rate (the reaction rate given a given time) can be determined by experiment. The nature of the reactant itself, an external factor: temperature, concentration, pressure, catalyst, light, laser, reactant particle size, contact area and reactant state, X-ray, gamma ray, surface area of X-rays, gamma rays, solid substances, and The contact area of the reactants, the concentration of the reactants can also affect the chemical reaction rate.
collision
collision theory, is from Germany's Max Trautz and UK William Lewis in 1916 and 1918 Submitted separately.
-
collision: Any chemical reaction occurs, the necessary reaction particles are close to the collision, and the reaction rate is proportional to the number of collisions.
-
activation energy: so-called activation energy is the minimum energy that can react to particles.
-
Effective collision: The so-called effective collision means that the energy of the collision has exceeds the activation energy, and the collision direction (bits) should be correct (the energy required for chemical reactions).
Factors affecting the reaction rate
In addition to the nature of the reactant, concentration, temperature and catalyst are also an important factor affecting the rate of reaction. The quick slowness of the gas reaction is also related to the pressure. Increase the concentration of the reactant, that is, the number of activated molecules in unit volume is increased, thereby increasing the number of effective collisions of the reactant molecules within the unit time, resulting in speeding of the reaction rate. Improve the reaction temperature, that is, the percentage of activation molecules, also increased the number of reactant molecules in units of time, resulting in acceleration of the reaction rate. Using a positive catalyst, the reaction process has been changed, and the activation energy required to react, and the reaction rate is accelerated. In chemical production, the reaction conditions are often controlled to accelerate the reaction rate to increase production. Sometimes it is necessary to take steps to slow the rate of reaction to extend the use of products.
